ORIGINAL ARTICLE
Hippokratia 2015, 19(1):41-46
Eleftheriadis T, Pissas G, Antoniadi G, Makri P, Liakopoulos V, Stefanidis I
Department of Nephrology, Medical School, University of Thessaly, Larissa, Greece
Abstract
Background: Urate through NOD-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome-dependent caspase-1 activation stimulates macrophages to secrete inteleukin-1β (IL-1β). Urate also enhances adaptive immunity indirectly through its effect on antigen presenting cells. In this study, the direct effect of urate on isolated primary human T-cells was evaluated.
Methods: Isolated T-cells were cultured with or without monosodium urate crystals in the presence or not of the NLRP3 inflammasome inhibitor glyburide. Activated cleaved caspase-1 was assessed by means of western blotting, whereas caspase-1 activity was measured colorimetrically in the cell lysates. IL-1β was measured in the supernatants by means of enzyme-linked immunosorbent assay. T-cell proliferation was assessed by means of bromodeoxyuridine labelling and immunoenzymatic detection.
Results: Urate induced caspase-1 activation and IL-1β release by T-cells. It also induced proliferation of T-cells. Glyburide inhibited urate-induced caspase-1 activation, IL-1β secretion and proliferation.
Conclusions: Urate, a well defined danger signal, stimulates directly human T-cells in a NLRP3 infmmasomela-dependent way. The subsequent IL-1β secretion could enhance inflammation, whereas expansion of T-cell clones could facilitate a subsequent adaptive immune response. Hippokratia 2015, 19 (1): 41-46.
Key words: Urate, T-cells, caspase-1, interleukin-1β, NLRP3, inflammasome
Corresponding author: Theodoros Eleftheriadis, MD, PhD, Department of Nephrology, Medical School, University of Thessaly, Neo ktirio, Mezourlo Hill, 41110 Larissa, Greece, tel: +302413501668, fax: +302413500242, e-mail: teleftheriadis@yahoo.com
Introduction
Urate is the end product of purine metabolism in humans and higher primates. Besides a byproduct of purine metabolism, urate also acts as a danger associated molecular pattern (DAMP)1. Monosodium urate (MSU) crystals, the etiological agent of gout, are recognized by the NOD-like receptor family, pyrin domain containing 3 (NLRP3) in macrophages leading to inflammasome formation. Once inflammasome is formed, caspase-1 is activated and converts pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) to active IL-1β and IL-18, respectively. Then these potent proinflammatory cytokines are secreted2.
The exact mechanism by which NLRP3 is activated by MSU crystals is not fully understood. Yet, the ability of NLRP3 to sense various structurally diverse stimuli led to the hypothesis that it does not recognize each stimulus individually but senses a common downstream event3. It seems that intracellular potassium depletion is such an event since it triggers NLRP3 activation, while inhibition of potassium efflux prevents NLRP3 dependent caspase-1 activation in response to MSU crystals4,5. However, the exact sequence of the events that could lead to intracellular potassium depletion needs further clarification.
Urate released by dying cells has been identified as a DAMP that induces sterile inflammation. The role of MSU crystals as an endogenous, non redundant danger signal has been confirmed in many models of sterile inflammation such as in the acetaminophen-induced hepatototoxicity model6 and in the bleomycin-induced lung injury model7.
Although uric acid plays a significant role in inflammation, less is known about its direct effect on the cells of adaptive immunity. Studies showed that MSU crystals promote immune rejection of tumors8, enhance T-cell immune response to antigens9, and play important role when ,aluminium salts are used as adjuvants, in order to enhance adaptive immunity response to vaccines10. However, these studies focused on the effect of uric acid on dendritic cells and not on its direct effect on T-cells. Interestingly, in a previous study, we showed that in a monocyte depleted population of peripheral blood mononuclear cells (PBMCs), that included T-cells, NK-cells and B-cells, urate crystals induce caspase-1 activation and IL-1β secretion11. In this study the direct effect of uric acid on isolated primary human T-cells, which are known to express NLRP312, was evaluated.
Materials and Methods
Subjects
Blood samples from 10 healthy volunteers (6 women/4 men, 22 to 47 years old) were collected. An informed consent was obtained from each individual enrolled into the study and the hospital ethics committee gave its approval to the study protocol.
T-cell isolation and culture
PBMCs were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation (Histopaque 1077, Sigma-Aldrich, St. Louis, MO, USA). Immediately after, T-cells were isolated from the PBMCs by negative selection. Non T-cells were indirectly magnetically labeled with a cocktail of biotin conjugated monoclonal antibodies and were depleted using the Pan T-cell Isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the isolated T-cells was tested and confirmed by means of flow cytometry. Afterwards isolated T-cells were counted via optical microscopy on a Neubauer plaque and cell viability was determined by trypan blue assay (Sigma-Aldrich).
T-cells were resuspended in RPMI 1640 medium with L-glutamine and 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and supplemented with 10% fetal bovine serum (Sigma-Aldrich) and antibiotic-antimycotic solution (Sigma-Aldrich). Cells were cultured with or without urate at a concentration of 10mg/dL (Sigma-Aldrich) and in the presence or not of the NLRP3 inhibitor glyburide (InvivoGen, San Diego, CA, USA) at a concentration of 25 mcg/mL. It should be noted that at the above concentration urate was precipitated, forming MSU crystals. All cultures were performed at 37oC in a humidified atmosphere containing 5% CO2.
Assessment of activated cleaved caspase-1
T-cells (1 x 106/well) were cultured in flat-bottom 12 well plates for 48 hours, with or without uric acid at a concentration of 10 mg/dL and in the presence or not of glyburide at a concentration of 25 mcg/mL. Equal numbers of T-cells were lysed using the T-PER tissue protein extraction reagent (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich and Roche Diagnostics). Protein was quantified via Bradford assay (Sigma-Aldrich) and western blotting was performed. Equal quantities of protein extracts (50mcg) from each sample were loaded for electrophoresis in sodium dodecyl sulfate (SDS) polyacrylamide gels (Invitrogen, Life Technologies, Carlsbad, CA, USA). Subsequently proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Invitrogen, Life Technologies). Blots were incubated with the primary antibody against cleaved caspase-1 (Asp297) (Cell Signaling Technology, Danvers, MA, USA) at a dilution of 1:1000 for 16 hours, followed by the secondary antibody anti-rabbit IgG, HRP-linked Antibody, (Cell Signaling Technology) at a dilution of 1:5000 for 30 min. Benchmark pre-stained protein ladder (Invitrogen, Life Technologies) was used as a marker. For normalization of the results the expression of the house-keeping gene β-actin was assessed using a specific antibody (Cell Signaling Technology) at a dilution of 1:5000. Bands were visualized by enhanced chemiluminescent detection using the LumiSensor Plus Chemiluminescent HRP Substrate Kit (GenScript, Piscataway, NJ, USA) and analysis was performed using the Image J software (National Institute of Health, Bethesda, MD, USA). A representative lane is depicted in Figure 1.
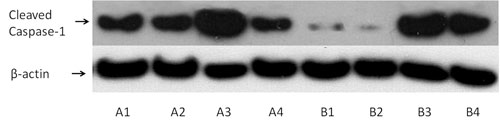
Figure 1. A representative western blotting lane of activated cleaved caspase-1 expression in primary human T-cells. T-cells were isolated from peripheral blood mononuclear cells (PBMCs) of ten healthy volunteers and treated as described below. Then western blotting performed in the extracted proteins. Two of the ten performed experiments are depicted. Capital letters A and B correspond to two different healthy volunteers. Number 1 refers to untreated T-cells, number 2 to T-cells treated with the NODlike receptor family, pyrin domain containing 3 (NLRP3) inhibitor glyburide, number 3 to T-cells treated with urate and number 4 to T-cells treated with both urate and glyburide.
Assessment of caspase-1 enzymatic activity and IL-1β secretion
T-cells (1 x 106/well) were cultured in flat-bottom 12 well plates for 48 hours, with or without uric acid at a concentration of 10 mg/dL and in the presence or not of glyburide at a concentration of or 25 mcg/mL. Caspase-1 enzymatic activity was assessed colorimetrically in T-cell protein extract. A kit based on the degradation of a caspase-1-specific peptide that is conjugated to the color reporter molecule p-nitroaniline (pNA) was used. The cleavage of the peptide by the caspase releases the chromophore pNA (Caspase -1 Colorimetric Assay, R&D Systems, Abingdon, UK).
IL-1β was measured in the supernatant by means of enzyme-linked immunosorbent assay (Human IL-1β Platinum ELISA, Bender MedSystems GmbH, Vienna, Austria). The sensitivity of the above ELISA kit is 0.3 pg/mL.
All these experiments were performed in duplicates and the results refer to the mean of the two measurements.
Assessment of T-cell proliferation
T-cells (1 x 105/well) were cultured in flat-bottom 96 well plates and treated with uric acid at a concentration of 10 mg/dL in the presence or not of glyburide at a concentration of 25 mcg/mL for 96 hours. T-cell proliferation was assessed by Cell Proliferation ELISA (Roche Diagnostics, Indianapolis, IN, USA) using bromodeoxyuridine (BrdU) labelling and immunoenzymatic detection according to the manufacturer’s protocol. All these experiments were performed in triplicates and the results refer to the mean of the three measurements.
Statistical Analysis
Normality of the evaluated variables was assessed and confirmed by one-sample Kolmogorov-Smirnov test. Sphericity assumption was evaluated by Mauchly’s test and if violated, degrees of freedom were corrected using Greenhouse-Geisser or Huynh-Feldt estimates of sphericity. Comparison of means was assessed by one-way repeated measures analysis of variance (ANOVA) followed by Bonferroni’s correction test. Results were expressed as mean ± standard deviation and p values lower than 0.05 were considered statistically significant.
Because in the case of western blotting, the caspase-1 activity and the BrdU assay results were expressed as optical densities (OD), p values were calculated by comparing the means of OD. Statistical analysis of relative to the controls OD values was avoided, in order to prevent violation of the prerequisite for normal distribution of the compared variables, when applying parametric statistical tests. However, the results were also expressed after normalization of means for the control group, according to the equation VariableX = (VariableX/Control groupX) x100.
Results
Urate crystals increase NLRP3-dependent activated cleaved caspase-1 and caspase-1 enzymatic activity in primary human T-cells
Urate crystals increase the quantity of activated cleaved caspase-1 in T-cells. The mean OD of the western blotting bands that correspond to the cleaved caspase-1 of urate treated T-cells was 18.06 ± 6.25 and significantly higher than the mean OD of these bands in untreated T-cells, which was 4.99 ± 4.53 (p<0.001). Thus, urate crystals increase cleaved caspase-1 in T-cells up to the 361.9% of its baseline level. The mean OD of the western blotting bands that correspond to the cleaved caspase-1 of glyburide treated T-cells was 7.11 ± 6.09 (that is at the 142.5% of the baseline) and were relatively the same with the mean OD of these bands in untreated T-cells (p=1.0). Thus the NLRP3 inhibitor glyburide does not affect the baseline level of cleaved caspase-1 in T-cells significantly. However, glyburide significantly decreases the quantity of the cleaved caspase-1 in urate treated T-cells. The mean OD of the western blotting bands that corresponds to the cleaved caspase-1 in T-cells treated with both urate and glyburide was 9.84 ± 3.18 (that is 197.2% of the baseline) and was significantly lower than the mean OD of these bands in T-cells treated only with urate (p=0.003). Interestingly, no statistical significance was detected regarding the quantity of cleaved caspase-1 between untreated T-cells and T-cells treated with both urate and glyburide (p=0.133), (Figure 2A).
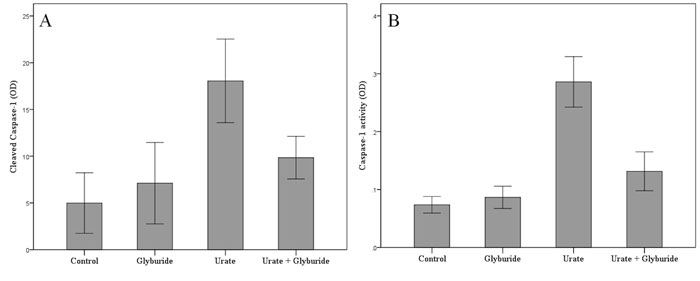
Figure 2. Urate crystals increase NOD-like receptor family, pyrin domain containing 3 (NLRP3)-dependent activated cleaved caspase- 1 (A) and caspase-1 enzymatic activity (B) in primary human T-cells. T-cells were isolated from peripheral blood mononuclear cells (PBMCs) of ten healthy volunteers and treated as described below. As revealed by western blotting urate crystals increase the quantity of activated caspase-1 in T-cells, whereas the NLRP3 inhibitor glyburide abrogates urate-induced increase of cleaved caspase- 1 in T-cells (A). As demonstrated by caspase-1 activity assay urate crystals increase the enzymatic activity of caspase-1 in Tcells, whereas the NLRP3 inhibitor glyburide reduces urate-induced increase of enzymatic activity of caspase-1 in T-cells (B). Error bars correspond to 95% confidence intervals of difference.
Besides the quantity of activated cleaved caspase-1, enzymatic activity of caspase-1 followed a similar pattern. Urate crystals increase the enzymatic activity of caspase-1 in T-cells. The mean OD corresponding to the caspase-1 activity of urate treated T-cells was 0.29 ± 0.07 being significantly higher than the respective mean OD in untreated T-cells, which was 0.07 ± 0.02 (p<0.001). Thus, urate crystals increase by four-fold the activity of caspase-1 in T-cells (414.3% of its baseline level). The mean OD that corresponds to the caspase-1 activity of glyburide treated T-cells was 0.09 ± 0.01 (128.6% of the baseline) and there was no attributable difference with the respective mean OD in untreated T-cells (p=1.0). Thus the NLRP3 inhibitor glyburide does not affect the caspase-1 activity in T-cells. However, glyburide significantly decreases the caspase-1 activity in urate treated T-cells. The mean OD that corresponds to the caspase-1 activity in T-cells treated with both urate and glyburide was 015 ± 0.10 (214.3% of the baseline) and was significantly lower than the respective mean OD in T-cells treated only with urate (p<0.001). Interestingly, when compared with untreated T-cells, caspase-1 activity remained higher in T-cells treated with both urate and glyburide (p=0.01), (Figure 2B).
Urate crystals increase NLRP3-dependent IL-1β secretion by primary human T-cells
Urate crystals increase IL-1β secretion by T-cells. In the supernatants of urate treated T-cells IL-1β concentration was 188.0 ± 34.8 pg/mL and was significantly higher than its concentration in the supernatants of untreated T-cells, which was 9.5 ± 4.8 pg/mL (p<0.001). Regarding baseline levels, urate crystals increase IL-1β secretion by T-cells up to 1978.9% of its baseline level. The IL-1β concentration in the supernatant of glyburide treated T-cells was 21.5 ± 6.7 pg/mL (that is at the 226.3% of the baseline) and was significantly higher than its concentration in the supernatants of untreated T-cells (p<0.001). Thus the NLRP3 inhibitor glyburide increases the baseline secretion of IL-1β in T-cells significantly. However, glyburide markedly decreases IL-1β concentration in the supernatants of urate treated T-cells. The concentration of IL-1β in the supernatants of T-cells treated with both urate and glyburide was 73.8 ± 5.4 pg/mL (that is 776.8% of the baseline) and was significantly lower than its concentration in the supernatants of T-cells treated only with urate (p<0.001). Interestingly, when compared with untreated T-cells, IL-1β concentration remained higher in the supernatants of T-cells treated with both urate and glyburide (p<0.001), (Figure 3).

Figure 3. Urate crystals increase NOD-like receptor family, pyrin domain containing 3 (NLRP3)-dependent interleukin (IL)-1ß secretion by primary human T-cells. T-cells were isolated from peripheral blood mononuclear cells (PBMCs) of ten healthy volunteers and treated as described below. Then IL-1ß was measured in the supernatants by means of ELISA. Urate crystals increase IL-1ß secretion by T-cells, whereas the NLRP3 inhibitor glyburide reduces urate-induced secretion of IL-1ß by T-cells. Error bars correspond to 95% confidence intervals of difference.
Urate crystals induce NLRP3-dependent proliferation in primary human T-cells
Urate crystals induce proliferation T-cells. The mean BrdU assay-derived OD in urate treated T-cells was 0.49 ± 0.13 and was significantly higher than the respective mean OD in untreated T-cells, which was 0.24 ± 0.04 (p<0.001). Proliferation of T-cells in the presence of urate crystals is increased two-fold (204.2% of its baseline level). The mean BrdU assay-derived OD in glyburide treated T-cells was 0.25 ± 0.07 (104.1% of the baseline) and did not differ significantly with the respective mean OD in untreated T-cells (p=1.0). Consequently, the NLRP3 inhibitor glyburide does not affect the proliferation of T-cells. However, glyburide significantly decreases the proliferation in urate treated T-cells. The mean BrdU assay-derived OD in T-cells treated with both urate and glyburide was 0.30 ± 0.07 (that is at the 125.0% of the baseline) and was significantly lower than respective mean OD in T-cells treated only with urate (p<0.001). Interestingly, when compared with untreated T-cells, proliferation remained higher in T-cells treated with both urate and glyburide (p=0.031), (Figure 4).
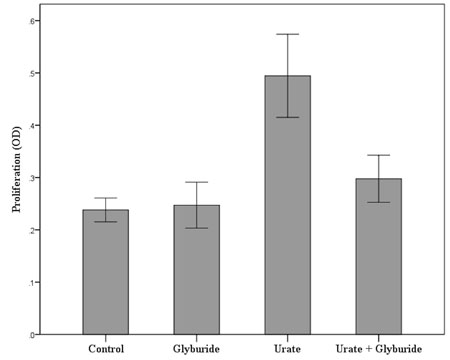
Figure 4. Urate crystals induce NOD-like receptor family, pyrin domain containing 3 (NLRP3)-dependent proliferation in primary human T-cells. T-cells were isolated from peripheral blood mononuclear cells (PBMCs) of ten healthy volunteers and treated as described below. As revealed by bromodeoxyuridine (BrdU) assay urate crystals induce T-cell proliferation, whereas the NLRP3 inhibitor glyburide reduces urate-induced T-cell proliferation. Error bars correspond to 95% confidence intervals of difference.
Discussion
Urate is a known DAMP. Traditionally it is thought that pathogen associated molecular patterns (PAMPs) and DAMPs are sensed by cells of the innate immune system that induce inflammation and/or shape a subsequent adaptive immune response13. The effect of urate on professional antigen presenting cells such as the macrophages and the dendritic cells has been extensively studied2,8-10. Also the role of NLRP3-inflammasome in urate-induced activation of these cells has been confirmed2,4,5. Interestingly, NLRP3 is expressed in T-cells as well12. The aim of this study was to evaluate the direct effect of urate on primary human T-cells, since the available data are scarce. For this reason we isolated and cultured T-cells from healthy volunteers.
It should be noted that at the designated concentration of 10 mg/dL urate was precipitated forming MSU crystals. It has been established previously, albeit in other cell types, that urate only in the form of MSU crystals and not in its soluble form enhances inflammatory response14.
In consensus with previous data from other cell types2, in isolated T-cells MSU crystals induce the same sequence of events. More precisely in T-cells, urate increased markedly the quantity of activated cleaved caspase-1 as well as its enzymatic activity. A significant increase of IL-1b was also noted in the same cellular context.
In order to elucidate if urate acts in T-cells through the known NLRP3 dependent caspase-1 activation pathway2, T-cells were treated with the NLRP3 inhibitor glyburide. Glyburide blocks the maturation of caspase-1 and pro-IL1-β by inhibiting potassium efflux15. Glyburide was shown to potently block the activation of NLRP3 inflammasome induced by PAMPs and DAMPs16,17. Glyburide works downstream of the purinergic receptor P2X7 but upstream of NLRP316. In isolated T-cells glyburide inhibited significantly both urate-induced increase of cleaved caspase-1 quantity and caspase-1 enzymatic activity. Moreover the increased secretion of IL-1β by T-cells due to urate treatment was markedly decreased by glyburide. Therefore like in professional antigen presenting cells, in T-cells the MSU crystals induce NLRP3 inflammasome-dependent caspase-1 activation and IL-1β maturation and secretion. The above results are quite interesting, since they confirm that a DAMP can act directly in T-cells, inducing IL-1β secretion and possibly promoting inflammation independently of cells of the innate immunity.
Notably, compared to untreated T-cells, western blotting revealed that there was a trend towards increased cleaved caspase 1 quantity in T-cells treated with urate and glyburide, which however did not reach statistical significance. However, compared to untreated T-cells, caspase 1 activity was slightly increased in T-cells treated with both urate and glyburide, albeit to a far less extent than in T-cells treated with urate alone. It is likely that at the used concentration glyburide does not inhibit the NLRP3 pathway completely.
Another appealing result of the present study is that MSU crystals induced T-cell proliferation in the absence of an antigen receptor specific stimulus. Once again T-cell proliferation is NLRP3 inflammasome-dependent considering glyburide reduced markedly urate-induced proliferation. This antigen independent T-cell proliferation may explain the observed adjuvanticity of urate crystals10, since expansion of T-cell clones could facilitate a subsequent adaptive immune response.
The exact mechanisms prior to NLRP3 activation have to be elucidated. Traditionally, it was thought that urate crystals and other solid structures can only induce NLRP3 activation in cells with phagocytic capacity. Antigen presenting cells internalize the solid structure into a phagosome that subsequently undergoes acidification and maturation to lysosome. However, due to the nature of its solid structure, the lysosome ruptures and releases cathepsin B into the cytoplasm. Cathepsin B signals potassium efflux and reactive oxygen species production activating the NLRP3 inflammasome4,18-20. Nevertheless, it has also been shown that MSU crystals and other solid structures can trigger cells without internalization. Atomic force microscopy studies confirmed that cholesterol-rich domains in the lipid membrane, also known as lipid rafts, have a natural affinity to MSU crystals. These domains are home to various immunoreceptor tyrosine-based activation motif (ITAM)-associated transmembrane proteins. The aggregation of cholesterol rich regions to MSU crystals increases the spatial density of such ITAM motifs such that the basal phosphorylation of these ITAMs spontaneously triggers intracellular signaling in an extracellular protein receptor-independent manner. In case of dendritic cells this pathway activates the spleen tyrosine kinase (Syk) signaling21. Considering the importance of these lipid units in T-cell activation22, the direct interaction of MSU crystals with T-cell membrane as a mechanism for NLRP3 dependent T-cell activation could not be excluded and deserves further evaluation.
In conclusion, urate, a well defined danger signal, stimulates directly human T-cells in a NLRP3 inflammasome-dependent way. The subsequent IL-1β secretion could enhance inflammation, whereas expansion of T-cell clones could facilitate a subsequent adaptive immune response.
Conflict of interest
Authors report no conflict of interest. Authors alone are responsible for the content and writing of the paper.
References
1. Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003; 425: 516-521.
2. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; 440: 237-241.
3. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10: 826-837.
4. Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007; 14: 1583-1589.
5. Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A. 2008; 105: 9035-9040.
6. Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010; 120: 1939-1949.
7. Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009; 179: 903-913.
8. Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004; 64: 5059-5062.
9. Ma XJ, Tian DY, Xu D, Yang DF, Zhu HF, Liang ZH, et al. Uric acid enhances T cell immune responses to hepatitis B surface antigen-pulsed-dendritic cells in mice. World J Gastroenterol. 2007; 13: 1060-1066.
10. Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008; 205: 869-882.
11. Eleftheriadis T, Pissas G, Karioti A, Antoniadi G, Golfinopoulos S, Liakopoulos V, et al. Uric acid induces caspase-1 activation, IL-1beta secretion and P2X7 receptor dependent proliferation in primary human lymphocytes. Hippokratia. 2013; 17: 141-145.
12. Petterson T, Mansson A, Riesbeck K, Cardell LO. Nucleotide-binding and oligomerization domain-like receptors and retinoic acid inducible gene-like receptors in human tonsillar T lymphocytes. Immunology. 2011; 133: 84-93.
13. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007; 81: 1-5.
14. Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010; 184: 6350-6358.
15. Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. The Journal of biological chemistry. 1999; 274: 36944-36951.
16. Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009; 187: 61-70.
17. Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009; 4: e6510.
18. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008; 9: 847-856.
19. Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008; 320: 674-677.
20. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010; 11: 136-140.
21. Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008; 29: 807-818.
22. Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010; 11: 90-96.
