ORIGINAL ARTICLE
Hippokratia 2015, 19(3):249-255
Kiroplastis K1, Fouzas I2,Katsiki E3, Patsiaoura K3, Daoudaki M2, Komninou A4, Xolongitas E2, Katsika E2, Kaidoglou K5, Papanikolaou V2
15th Surgical Department, Aristotle University of Thessaloniki, Hippokratio General Hospital, 2Division of Transplantation, Department of Surgery, Aristotle University of Thessaloniki, Hippokratio General Hospital, 3Department of Pathology, Hippokratio General Hospital, 4School of Veterinary Medicine, Aristotle University of Thessaloniki, 5Department of Histology Embryology, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Abstract
Background: Liver regeneration is vital for the survival of patients submitted to extensive liver resection as a treatment of hepatocellular carcinoma (HCC). Sorafenib is a multikinase inhibitor of angiogenesis and cell division, both of which are integral components of liver regeneration.We investigated the effect of preoperative treatment with sorafenib, a drug used for the treatment of HCC, on liver regeneration and angiogenesis in healthy rats, after two-thirds partial hepatectomy (PH2/3).
Methods: In total 48 Wistar rats received intragastric injections of sorafenib (30 mg/kg/d) or vehicle, underwent PH2/3, and were sacrificed at 48, 96 or 168 hours after that. The regenerative index of the liver remnant was studied, as well as the mitotic index. DNA synthesis and angiogenesis were estimated by immunohistochemistry for the Ki-67 and CD34 antigens, respectively.
Results: Sorafenib reduced significantly the regenerative index at all time points but not the mitotic index at 48, 96 or 168 hours. Deoxyribonucleic acid (DNA) synthesis and angiogenesis were not affected significantly either.
Conclusions: Sorafenib, when administered preoperatively, reduces incompletely and transiently the regeneration of the liver after PH2/3 in rats. This could mean that sorafenib can be used as neoadjuvant treatment of patients with HCC prior to liver resection, but further experimental and clinical studies are needed to establish the safety of this treatment. Hippokratia 2015; 19 (3): 249-255.
Key words: Sorafenib, partial hepatectomy, angiogenesis, liver regeneration, hepatocellular carcinoma, liver transplantation
Corresponding author: Ioannis Fouzas, MD, PhD, Associate Professor of General and Transplant Surgery, Division of Transplantation, Department of Surgery, Aristotle University Medical School, Hippokratio Hospital, 49, Konstantinoupoleos Ave, Thessaloniki, Greece, 54642, tel: +302310992889, fax: +302310855566, e-mail: ifouzas@auth.gr
Introduction
Liver has the unique ability to regenerate and thereby restore its original mass after tissue loss1. This regenerative capacity of the liver is considered to be vital for the survival of patients submitted to extensive liver resection for the treatment of malignant liver tumors. Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver, the fifth most common type of cancer worldwide, and the third cause of cancer mortality2,3. Treatment options for HCC are generally selected taking into account the tumor stage, liver function and patient performance status and include liver transplantation, liver resection, ablation, chemoembolization and systemic chemotherapy4. Liver transplantation and liver resection are considered as potentially curative treatments and are the standard of care for patients with early-stage HCC4. Patients with advanced HCC and Child–Pugh A liver disease, treatment with sorafenib, an orally active multikinase inhibitor with antiangiogenic and antiproliferative properties, resulted in a survival extension5. Additionally there is an increasing number of reports of patients with HCC treated with sorafenib, as neoadjuvant or adjuvant therapy before or after liver resection6. Therefore, the effect of sorafenib on liver regeneration could be crucial for the survival of these patients.
Sorafenib is an inhibitor of serine/threonine kinases as well as the tyrosine kinases of vascular endothelial growth factor receptor-2,-3 (VEGFR-2, VEGFR-3), platelet-derived growth factor receptor (PDGFR), FMS-related tyrosine kinase-3 (FLT-3), Proto-oncogene-tyrosine-protein-kinase-receptor RET, and proto-oncogene c-Kit7,8. The molecular pathways inhibited by sorafenib augment angiogenesis and cell division, both of which are integral components of liver regeneration1. This inhibition of vital processes by sorafenib could impair regeneration. The aim of the present study was to investigate how the preoperative treatment with sorafenib influences liver regeneration and angiogenesis in healthy rats, after two-thirds partial hepatectomy (PH2/3).
Materials and methods
Materials
Sorafenib (Eton Bioscience, Inc. 5820, San Diego, CA, USA) was dissolved in a mixture of 50% Cremophor (Sigma-Aldrich, St. Louis, MO, USA), 50% ethanol, diluted with water (12.5% Cremophor/12.5% ethanol/75% water) and administered to rats, orally, in daily basis in a volume of 250 μL/100g body weight. Monoclonal mouse anti-rat Ki-67 specific antibody (clone MIB-5, isotype IgG1, Dako®, Fort Collins, Colorado, USA) and anti-CD34 antibody (QBEND-10, Dako®, Fort Collins, Colorado, USA) were utilized.
Animals
In total 48 specified pathogen free, adult, male Wistar rats, weighing 200–250g were obtained from the Department of Physiology, of the Veterinary Medicine School, of Aristotle University of Thessaloniki, Greece, and were housed in a 12-hour light-dark cycle where temperature, humidity and ventilation were controlled as per international standards. They were fed with standard pellet chow and water ad libitum. All animals were acclimated for two weeks prior to experimentation. This study was approved by the local committee for Care and Use of Laboratory Animals (Ref.No. 13/14276 (09/13/09) Veterinary Department of Perception, Medicines and Applications, Veterinary Division, Prefecture of Thessaloniki, Greece). All operations and postoperative care of animals were performed in the Division of Surgery, Companion Animal Clinic, School of Veterinary Medicine of the Aristotle University of Thessaloniki.
Group assignment
Animals were allocated to two groups, which were further divided into three subgroups, consisting of 8 animals each. Animals in group 1 received placebo for Sorafenib by gavage for 14 days preoperatively, underwent partial hepatectomy and were sacrificed at 48, 96, and 168 hours postoperatively (groups 1a, 1b, and 1c, respectively). Animals in group 2 received 30 mg/kg of sorafenib by gavage for 14 days preoperatively, underwent partial hepatectomy and were sacrificed at 48, 96 , and 168 hours postoperatively (groups 2a, 2b, and 2c, respectively).
Surgical procedure
All animals were operated under inhalation anesthesia. Induction to anesthesia was performed in a plastic cylinder with isoflurane 4% concentration and maintenance to anesthesia was achieved with nasal mask with isoflurane 2% concentration. PH2/3 was performed as described by Higgins and Anderson9. A bilateral subcostal incision was performed and the median and left liver lobes were mobilized, ligated and excised. The abdominal wall was closed using a continuous absorbable suture and the skin with interrupted non-absorbable sutures. The excised liver lobes were weighed on an electronic scale. On reoperation, the previous surgical incision was opened, and the aorta was cannulated so as the animals were exsanguinated to death. Finally, the remaining hepatic attachments were divided, and the liver remnant was removed and weighed.
Hepatic regeneration rate
The rate of regeneration of the liver following hepatectomy was calculated according to the Kwon’s formula10 from the expression:
Hepatic regeneration rate (%) = D/Ex100 (E = R/0.7)
D: liver weight per 100g of body weight, on the day they were sacrificed
E: estimated liver weight per 100g of body weight before hepatectomy
R: liver weight resected at PH2/3
Light microscopy observations and hepatocyte proliferative activity
Liver tissue samples from the caudate lobe were obtained at the time of death. They were formalin-fixed and paraffin-embedded, then cut into 4 μm-thick sections and Hematoxylin and eosin stained. Hepatocyte proliferative activity was estimated by counting the mitosing hepatocytes in 50 consecutive high power fields (HPF: x400). Cells in prophase before the dissolution of nuclear membranes and late telophase were excluded.
Immunohistochemistry
Paraffin-embedded tissue sections of all specimens were prepared and labelled with Ki-67 specific monoclonal mouse antibody (1:100 dilution) and CD-34 specific monoclonal rabbit antibody (1:50 dilution) in an automated fashion (Bond, Leica, Germany).
Microvessel Density (MVD)
The MVD was assessed using CD34 staining. Five areas of the most intensive neovascularization were selected at x40 magnification throughout all the sections. Microvessels of these areas were counted at x200 magnification. The MVD was evaluated based on the recommendation of Gasparini and Harris11. Any brown-stained endothelial cell or endothelial cell cluster clearly separated from neighboring microvessels, hepatocytes and connective elements were considered to be countable microvessels.
Statistical analysis
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) for Windows version 17.0 (SPSS Inc. Chicago, USA). All values were expressed as means ± standard deviation (SD). The statistical significance was determined either by the student two-tailed t-test (parametric values) or Mann–Whitney U test (nonparametric values) and p <0.05 was considered to be significant.
Results
Regenerative index
Sorafenib significantly reduced the restoration of the liver remnant at 48, 96, and 168 hours after PH2/3 (Figure 1). The mean regenerative index in group 1a was 67.25 ± 2.024 vs 59 ± 1.51 in group 2a (p =0.007), in group 1b 80.88 ± 1.39 vs 70.00 ± 1.07 in group 2b (p <0.0001), and in group 1c 88.88 ± 1.62 vs 82.25 ± 1.86 in group 2c (p =0.0177).
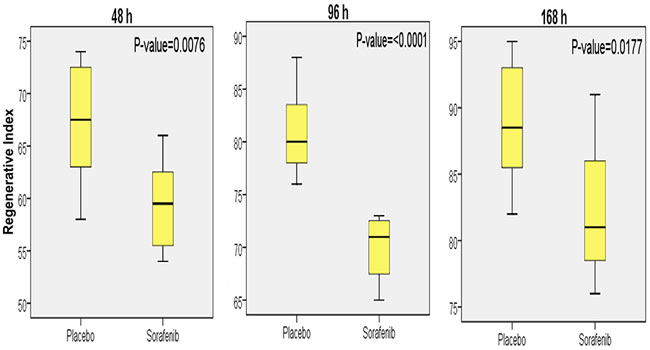
Figure 1: Mean regenerative rate ± standard deviation, and statistical analysis for the groups of animals that received either sorafenib or placebo (vehicle) and were sacrificed at 48, 96, or 168 hours after two-thirds partial hepatectomy (PH2/3).
Optical microscopy and mitotic index
Light microscopy of tissue specimens from all groups revealed the characteristic changes of liver regeneration such as hepatocyte swelling, microvesicular steatosis, dividing nuclei, visible nucleoli and bi-columnar hepatocyte sheets. These changes were more prominent in the animals that received only the vehicle (Figure 2).

Figure 2: Cell proliferation was estimated with histological staining using Hematoxylin and Eosin kit. Placebo group images at 48 hours (1a), 96 (1b) and 168 (1c) hours after two-thirds partial hepatectomy (PH2/3) and sorafenib group images at 48 hours (2a), 96 (2b), and 168 (2c) hours after PH2/3 respectively (x400).
Sorafenib considerably reduced proliferation of hepatocytes at 48 and 96 hours after PH2/3, but did not have any effect at 168 hours (Figure 3). The mean mitotic index in group 1a was 69.63 ± 6.99 vs 45.63 ± 4.26 in group 2a (p =0.01), in group 1b 24.63 ± 2.35 vs 14.75 ± 1.68 in group 2b (p =0.004) and in group 1c 4.88 ± 0.61 vs 3.88 ± 0.51 in group 2c (p =0.23).

Figure 3: Mean mitotic index ± standard deviation, and statistical analysis for the groups of animals that received either sorafenib or placebo (vehicle) and were sacrificed at 48, 96, or 168 hours after two-thirds partial hepatectomy (PH2/3).
DNA synthesis
Hepatocellular DNA synthesis was estimated using immunohistochemical staining for the Ki-67 antigen as described by Gerlach et al12. Ki-67 is expressed during all active phases of the cell cycle (G1, S, G2, and mitosis phases), but not in resting (G0) cells13. Sorafenib did not reduce DNA synthesis at 48, 96, and 168 hours after PH2/3 (Figure 4, Figure 5). The mean labelling index with anti ki-67 monoclonal antibody in group 1a was 42.88 ± 3.94 vs 35.00 ± 1.88 in group 2a (p =0.093), in group 1b 26.25 ± 2.79 vs 20.63 ± 3.05 in group 2b (p =0.12), and in group 1c 3.38 ± 0.32 vs 4.13 ± 0.47 in group 2c (p =0.21).
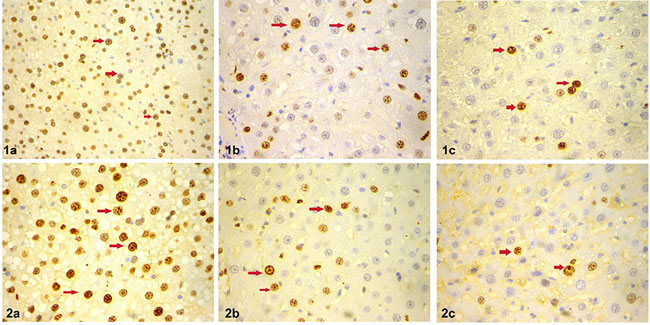
Figure 4: Hepatocellular DNA synthesis estimation using immunohistochemical staining for the Ki-67 antigen. Placebo group images at 48 hours (1a), 96 (1b), and 168 (1c) hours after two-thirds partial hepatectomy (PH2/3) and Sorafenib group images at 48 hours (2a), 96 (2b), and 168 (2c) hours after PH2/3 respectively. Arrows indicate Ki-67 positive nuclei (x200).
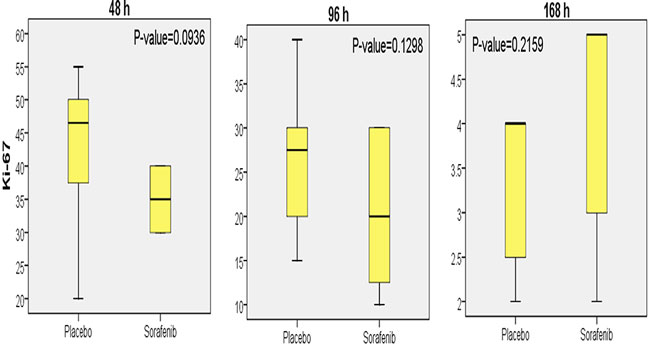
Figure 5: Mean Ki-67 labelling index ± standard deviation, and statistical analysis for the groups of animals that received either sorafenib or placebo (vehicle) and were sacrificed at 48, 96, or 168 hours.
Neoangiogenesis
Liver angiogenesis during regeneration is characterised by changes in the phenotype of sinusoidal endothelial cells, which assume the features of capillary endothelial cells. This process known as sinusoidal capillarization (SC) can be routinely assessed by CD34 immunostaining, resulting negative in normal liver sinusoids and positive in the capillarised ones14.
Sorafenib did not reduce neoangiogenesis at any time point (Figure 6, Figure 7). The mean labelling index with anti CD34 monoclonal antibody in group 1a was 65.00 ± 4.62 vs 55.00 ± 4.62 in group 2a (p =0.087), in group 1b 40.00 ± 4.63 vs 26.25 ± 5.88 in group 2b (p =0.0147) and in group 1c 11.25 ± 2.05) vs 15.63 ± 2.9 in group 2c (p =0.28).

Figure 6: Sinusoidal capillarization assessment by CD34 immunostaining, resulting negative in normal liver sinusoids and positive in the capillarised ones. Placebo group images at 48 hours (1a), 96 (1b), and 168 (1c) hours after two-thirds partial hepatectomy (PH2/3) and Sorafenib group images at 48 hours (2a), 96 (2b), and 168 (2c) hours after PH2/3 respectively (x200).

Figure 7: Mean CD34 labelling index ± standard deviation, and statistical analysis for the groups of animals that received either sorafenib or placebo (vehicle) and were sacrificed at 48, 96, or 168 hours.
Discussion
The effect of sorafenib on liver regeneration has been previously studied with conflicting results. Hora et al15 demonstrated an inhibitory effect on liver regeneration in mice receiving sorafenib after PH2/3; while no effect was seen if the treatment was stopped one day before surgery. In contrast, Kurniali et al16 assessed cell proliferation by flow cytometry on isolated liver cells from perfused livers stained with anti-bromodeoxyuridine and antiKi-67; in sorafenib-treated mice at 48, 96, or 360 hours after PH. They found no inhibiting effect on liver weight, DNA synthesis, or cellular proliferation if sorafenib was given after liver resection. More recently, Andersen et al17 reported that the pre and postoperative administration of 15 mg/Kgr/day of sorafenib to rats after PH was associated with a significant impairment of liver weight gain, regeneration rates and hepatocyte proliferation. Also, Mollbrink et al18 studied the effect of sorafenib up to 14 days after PH in rats and reported a prolongation of liver regeneration characterized by an initial reduction and then by recovery of the regenerative and labelling indices with bromodeoxyuridine and Ki-67.
The major finding of this study is that sorafenib when administered at a dose of 30 mg/Kg/day preoperatively, reduces incompletely and transiently the regeneration of the liver after PH2/3. More specifically, sorafenib significantly reduces the restoration of the liver remnant at 48, 96, and 168 hours after PH2/3. This inhibitory effect is also found in the mitotic index but only at 48 and 96 hours and not at 168 hours after PH. DNA synthesis as assessed with the Ki-67 labelling index was not considerably reduced at 48, 96, and 168 hours after PH2/3. Similarly, sorafenib did not reduce the neoangiogenesis of liver regeneration as assessed by the CD34 labelling index, at any time-point after PH2/3.
Liver regeneration is a complex process, which depends on the activation of several complementary growth signal pathways. The RAS/RAF/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, is activated during liver regeneration and in turn activates ERK1/219. Growth factors such as hepatocyte growth factor (HGF), endothelial growth factor (EGF), transforming growth factor-α (TGF-α) and different cytokines including interleukin-6 and tumor necrosis factor alpha (TNF-α), augment ERK1/2 activation20. Sorafenib inhibits the serine/threonine kinase activity of RAF in the RAF/MEK/ERK signalling pathway and the receptor tyrosine kinase activity of the VEGFR-28,21. The significance of ERK1/2 activation in hepatocyte proliferation is supported by previous reports22 while disputed by others23. More recently, in conditional Met-mutant mice only mild effects on liver regeneration after hepatectomy were found, despite low ERK1/2 levels24. Additionally, there are other signal transduction pathways involved in the activation of liver regeneration, such as c-Jun N-terminal protein kinases (JNK1/2), p38 mitogen-activated protein kinases and the phosphoinositide-3 kinase (PI3-kinase)23,25. This data suggests a redundancy of the activation mechanisms rather than exclusive roles of selected pathways and could explain the findings of our study that sorafenib reduces incompletely and transiently hepatocyte proliferation PH2/3.
Liver regeneration is characterized by hepatocyte proliferation but it also depends on endothelial cell proliferation and angiogenesis26,27. VEGF is considered a principal mediator of angiogenesis and also participates in the induction of growth factors in the regenerating liver28. Endothelial cell proliferation and survival is mediated by the VEGFR-229, the tyrosine kinase activity of which is inhibited by sorafenib8,21. However, mice genetically heterozygous for deficiency in the VEGFR have a normal endothelial cell proliferation, pointing at several pathways regulating endothelial cell proliferation30. This explains our finding that sorafenib did not reduce neoangiogenesis of liver regeneration significantly, as assessed by the CD34 labeling index, neither at 96 hours after PH2/3, nor at 48 and 168 hours.
Our finding that sorafenib, when administered at a dose of 30 mg/Kg preoperatively, reduces incompletely and transiently the regeneration and angiogenesis of the liver after PH2/3 cannot be translated into the clinical setting. In humans, such incomplete and transient inhibition could become dangerous. In a clinical setting, sorafenib could be used as a neoadjuvant treatment for an extended time period before resection or transplantation, which may compromise liver mass restoration to a higher degree than demonstrated in the present study; in which sorafenib was given for only two weeks before surgery.
Sorafenib is the only FDA-approved systemic chemotherapeutic agent for the treatment of advanced HCC at this time31. It appears to be safe if given after liver transplantation to prevent tumor recurrence32. Patients with HCC exceeding Milan criteria may benefit if treated with sorafenib as it delays tumor recurrence after liver transplantation and prolongs survival33. There are further ongoing Phase 2 clinical trials studying the safety and efficacy of the treatment34. However, when administered before transplantation, it was reported to increase the risk of postoperative biliary complications35. The clinical experience of treatment with sorafenib in patients with HCC, as neoadjuvant or adjuvant therapy before or after liver resection, is very limited. There are reports stating that sorafenib can downstage HCC, presenting a bridge to surgical treatment6, without compromising patients’ safety36.
In conclusion, our study shows that sorafenib reduces incompletely and transiently the regeneration and angiogenesis of the liver after PH2/3 in rats. This could mean that sorafenib can be used as neoadjuvant treatment in patients with HCC prior to liver resection. Nevertheless, to recommend such treatment as widespread application, further experimental and clinical studies with randomized trials have to verify their safety and determine their efficacy.
Conflict of interest
Authors declare no conflict of interest.
References
1. Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013; 3: 485-513.
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics CA Cancer J Clin. 2011; 61: 69-90.
3. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379: 1245-1255.
4. Daoudaki M, Fouzas I. Hepatocellular carcinoma. Wien Med Wochenschr. 2014; 164: 450-455.
5. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359: 378-390.
6. Barbier L, Muscari F, Le Guellec S, Pariente A, Otal P, Suc B. Liver resection after downstaging hepatocellular carci-noma with sorafenib. Int J Hepatol. 2011; 2011: 791013.
7. Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006; 66: 11851-11858.
8. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibi-tor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008; 7: 3129-3140.
9. Higgins GM, Anderson RM. Experimental pathology of the liver. I Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931; 12: 186-202.
10. Kwon AH, Uetsuji S, Yamamura M, Hioki K, Yamamoto M. Effect of administration of fibronectin or aprotinin on liver re-generation after experimental hepatectomy. Ann Surg. 1990; 211: 295-300.
11. Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995; 13: 765-782.
12. Gerlach C, Sakkab DY, Scholzen T, Dassler R, Alison MR, Gerdes J. Ki-67 expression during rat liver regeneration after par-tial hepatectomy. Hepatology. 1997; 26: 573-578.
13. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182: 311-322.
14. Guido M, Pizzi M, Sacerdoti D, Giacomelli L, Rugge M, Bolognesi M. Beyond scoring: a modern histological assessment of chronic hepatitis should include tissue angiogenesis. Gut. 2014; 63: 1366-1367.
15. Hora C, Romanque P, Dufour JF. Effect of sorafenib on murine liver regeneration. Hepatology. 2011; 53: 577-586.
16. Kurniali PC, O’Gara K, Wang X, Wang LJ, Somasundar P, Falanga V, et al. The effects of sorafenib on liver regeneration in a model of partial hepatectomy. J Surg Res. 2012; 178: 242-247.
17. Andersen KJ, Knudsen AR, Kannerup AS, Sasanuma H, Nyengaard JR, Hamilton-Dutoit S, et al. Sorafenib inhibits liver re-generation in rats. HPB (Oxford). 2013; 15: 944-950.
18. Mollbrink A, Augsten M, Hultcrantz R, Eriksson LC, Stål P. Sorafenib prolongs liver regeneration after hepatic resection in rats. J Surg Res. 2013; 184: 847-854.
19. Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006; 43: S45-S53.
20. Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004; 279: 34530-34536.
21. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogene-sis. Cancer Res. 2004; 64: 7099-7109.
22. Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, et al. The mitogen-activated protein kinase ki-nase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999; 19: 6003-6011.
23. Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, et al. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997; 17: 3556-3565.
24. Borowiak M, Garratt AN, Wustefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regen-eration. Proc Natl Acad Sci U S A. 2004; 101: 10608-10613.
25. Coutant A, Rescan C, Gilot D, Loyer P, Guguen-Guillouzo C, Baffet G. PI3K-FRAP/mTOR pathway is critical for hepatocyte proliferation whereas MEK/ERK supports both proliferation and survival. Hepatology. 2002; 36: 1079-1088.
26. Drasdo D, Hoemhe S, Hengstler JG. A Quantiative Mathematical Modeling Approach to Liver Regeneration. Haussinger D (ed). Liver regeneration. De Gruyter, Berlin, 2011, 159-173.
27. Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, et al. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002; 236: 703-711; discussion 711-712.
28. Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, et al. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol. 2004; 40: 305-312.
29. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endotheli-al cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR acti-vation. J Biol Chem. 1998; 273: 30336-30343.
30. Shergill U, Das A, Langer D, Adluri R, Maulik N, Shah VH. Inhibition of VEGF- and NO-dependent angiogenesis does not impair liver regeneration. Am J Physiol Regul Integr Comp Physiol. 2010; 298: R1279-R1287.
31. Shetty K, Dash C, Laurin J. Use of adjuvant sorafenib in liver transplant recipients with high-risk hepatocellular carcinoma. J Transplant. 2014; 2014: 913634.
32. Saab S, McTigue M, Finn RS, Busuttil RW. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and efficacy. Exp Clin Transplant. 2010; 8: 307-313.
33. Huang L, Li GM, Zhu JY, Li Z, Li T, Leng XS. Efficacy of sorafenib after liver transplantation in patients with primary he-patic carcinoma exceeding the Milan criteria: a preliminary study. Onco Targets Ther. 2012; 5: 457-462.
34. Jonsson Comprehensive Cancer Center. A Phase II Randomized Multicenter Placebo-Controlled Blinded Study of Sorafenib Adjuvant Therapy in High Risk Orthotopic Liver Transplant (OLT) Recipients With Hepatocellular Carcinoma (HCC). April 8, 2015; Available at: https://clinicaltrials.gov/show/NCT01624285;, last accessed: 19/4/2015.
35. Truesdale AE, Caldwell SH, Shah NL, Argo CK, Al-Osaimi AM, Schmitt TM, et al. Sorafenib therapy for hepatocellular car-cinoma prior to liver transplant is associated with increased complications after transplant. Transpl Int. 2011; 24: 991-998.
36. Barbier L, Fuks D, Pessaux P, Muscari F, Le Treut YP, Faivre S, et al. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann Surg Oncol. 2013; 20: 3603-3609.
